| Location: Home >> News >> Research Progress | ||||
| Research Progress | ||||
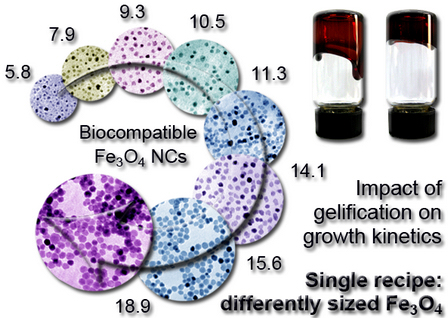
| Gelification: an Effective Measure for Achieving Differently Sized Biocompatible Fe3O4 Nanocrystals through a Single Preparation Recipe http://www.gaomingyuan.com Wednesday, November 16, 2011 10:00 |
||||
 |
||||
Inorganic nanocrystals exhibit unique particle size dependent physical properties because of the quantum confinement effect and the nanometer size effect. Although great success has been achieved over the past decades in regulating the particle size of various types of inorganic nanocrystals synthesized through different synthetic principles, developing new synthetic routes and further exploring the mechanisms for delicate control over the particle size remain hot subjects for wet-chemical synthesis of inorganic nanocrystals, especially for magnetic iron oxide nanocrystals due to their bright future in nanomedicine.
Gao’s group explored a simple and easy method to obtain biocompatible nanocrystals in-situ by the thermal decomposition approach, i.e., the one-pot reaction. The prepared magnetite nanocrystals show great effects on in vivo tumor detection as a novel type of dual-modality molecular probe (Chem. Mater. 2004, 16, 1391; Angew. Chem. Int. Ed. 2005, 44, 123; Adv. Mater. 2005, 17, 1001; Adv. Mater. 2006, 18, 2553;Mol. Pharm. 2009, 6, 1074;J. Phys. Chem. C 2010, 114, 21270.). Meanwhile, our gel-related research in Cu(acac)2/In(acac)3-in-dodecanethiol system show that the gelification phenomenon has a strong impact on the growth of the resultant Cu–In–S nanocrystals formed through successive pyrolysis of Cu(acac)2 and In(acac)3 precursors (J. Am. Chem. Soc. 2008, 130, 13152.). On the basis of the above research achievements, the size of magnetite nanocrystals can be effectively regulated simply by manipulating the gelification degree through aging the stock solutions with the same preparation recipe. Theoretical analysis and numerical simulations on the particle growth behavior suggest that the gelification of the reaction system, intrinsically reduces the thermal decomposition rate constants of the Fe precursors, and eventually gives rise to the gelification-associated size regulation effect for magnetite nanocrystals by altering their growth kinetics. The research results have been accepted by the Journal of the American Chemical Society. During this period, I have learnt much from Prof. Gao. What impressed me most is his patient instruction and help. To simulate the growth theory of nanocrystals, Prof. Gao looked for experts in this field for me and try his best to help me. This helpful work makes my research much better in quality and in scientific sense. Thanks to his patient guidance, my scientific quality has been trained, and also my abilities on communication and cooperation have been greatly improved. He makes himself an example on how to become a great scientist. One thing that impressed me most is that Prof. Gao patiently illustrate the difference between the word “stage” and “process”, and which one is more accurate in describing the experimental phenomenon. I will always remember his guidance on how to find and catch novel ideas, how to write a scientific paper and form one’s own style. These priceless experience and words will always be kept in my mind and guide me in my own scientific road. Qiaojuan Jia et al. |
||||